Rachel du Preez Winner -Technical Article - 2022
SOIL ACIDITY AND THE REMEDIATION THEREOF IN CROP PRODUCTION
Soil acidity is considered one of the most limiting factors in crop production worldwide. Declining soil fertility due to acidification is a combination of unawareness and financial restraints.
Soil acidification leads to a complex chemical change in soil, such as the increase in toxic levels of aluminium and manganese, inhibition of microbial processes, decrease in cation exchange capacity, decrease in accessibility of phosphorus and solubility of molybdenum and boron. Acidic soils are also stripped from macronutrients such as potassium, magnesium and calcium. The most evident problems with soil acidity are the decrease in root growth, the inability of plants to use water efficiently and to absorb plant nutrients in sufficient quantities. This contributes to lower productivity, lower yields, and ultimately a loss for the producer (Du Toit, 2019).
Liming applications form the basis for healthy crop production and economically food production. Therefore, a fixed lime application program is required to stabilize the chemical soil properties and to maintain it.
The purpose of this article is to provide scientifically detailed and practically valuable answers to questions such as: "What exactly soil acidity is?", "What the cause is?", "How does it impact crops?" and "How is it managed best?"
What is soil acidity?
An important property of soil is the soil reaction, thus whether the soil is acidic, neutral, or alkaline. The soil reaction is measured as pH and is also called active acidity. The acidity of soil can be considered healthy for agricultural crop production at a pH(KCl) between 5.5 and 6.5.
Clays and organic matter in the soil have a negative charge. In soil that is not acidic, this negative charge is balanced by the positive charge on plant nutrients, such as calcium (Ca2+), magnesium (Mg2+) and potassium (K+). When soil acidifies, concentrations of adsorbed hydrogen (H+) and aluminium (Al3+) increase and take the place of calcium, magnesium, and potassium on the clay and organic matter’s exchange complex (Figure 1) (Grain SA, 2013). This property is also called exchangeable acidity.

Figure 1: Increasing saturation of a clay particle with acidic cations (Al3+) and (H+) with increasing soil acidity from left to right (Grain SA, 2013).
Under non-acidic conditions, aluminium is embedded in the clay particles. As acidity increases, clay edges start to dissolve, releasing aluminium into the soil solution which is the most critical growth-limiting factor for crop production in acidic soils.
Causes of soil acidity?
Soils that develop from weathered granite, with a light texture (sandy to sandy loam) are more likely to be acidic than soils develop from shale, with a finer texture (loamy to sandy clay loam) or limestone (calcareous soil) (Du Toit, 2019).
There are four key reasons for soil to acidify: rainfall and leaching, acidic parent material, decaying of organic matter, and harvesting of high-yield crops (Grain SA, 2013). Acidic soils are common in high rainfall regions where basic cations have been leached from the soil, thus an increase in the H+-ion concentration and a decrease in the soil fertility. Deep sandy soils on higher-lying parts of the landscape, which are well-drained, are more likely to acidify than poorly drained soils in low-lying parts where the basic cations accumulate.
Accelerated decomposition of soil organic matter causes H+-ions to be released into the soil solution through the dissociation of organic acids (carboxylic and phenolic acids) as a result of tillage (Noble Research Institute, 1999).
Harvesting of high-yield crops plays the most significant role in soil acidification. During the growing season, crops absorb basic elements such as calcium, magnesium, and potassium to satisfy their nutritional requirements. As crop yields increase, as a result of high nitrogen fertilizer applications, more of these basic nutrients are removed from the soil.
Impact of soil acidity on crops?
Most crops and microorganisms function best at a neutral to slightly acidic pH(KCl) varying from 5.5 to 6.5. At the required pH, the nutrient accessibility for plant uptake and microbial activity is optimal (Figure 2).
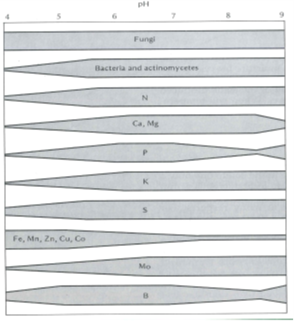
Figure 2: The relationship between the availability of plant nutrients, microbial activity, and pH (Brady & Weil, 2002).
In alkaline soil, several microelements (Fe, Mn, Zn, Cu, Co, and B) are inaccessible for plant uptake and deficiencies might occur. Mo and B are inaccessible in acidic soil, whereas Fe and Mn can occur in toxic concentrations. Phosphorus is less accessible in both strongly alkaline and acidic soils (Brady & Weil, 2002).
High concentrations of aluminium and manganese are released into the soil solution from clays as the soil acidifies. These high levels of Al and Mn are toxic to plant roots, with a result in poor and abnormal root development. Roots become thick and stubby with little to no development of essential fine roots (Grain SA, 2013). As a result, crops are unable to absorb sufficient amounts of water and nutrients and will appear stunted and exhibit nutrient deficiency symptoms. Depleted levels of calcium and magnesium are likely to occur in acidic soils and may establish a limitation to plant growth. The combination of high aluminium and low calcium levels in the subsoil is an extensive yield-limiting factor in grain-producing areas of South Africa.
Not all crops respond in a similar way to acidic soil conditions but vary widely in their tolerances to soil acidity (Du Plessis, 2019). Severe soil acidity has been shown to limit the growth of all species, including the highly tolerant ones. Thus, the management of soil acidity is an essential component of sustainable production practices, regardless of the crop grown.
The soil reaction (pH) is one of the most important chemical parameters in measuring soil's general chemical health. This significantly affects plant growth and the absorption of plant nutrients. The maintenance of an optimal pH is of great importance to the producer and has a significant influence on the sustainable use of this essential resource (Du Plessis, 2019).
Management practices for soil acidity?
In an agricultural context, lime refers to any product whose calcium and magnesium compounds can neutralize soil acidity. Calcium and magnesium carbonate are most commonly used. Dolomitic lime (CaMg(CO3)2) contains at least 15% magnesium carbonate, while calcitic lime (CaCO3) contains less (Fertasa Fertilizer Handbook, 2018).
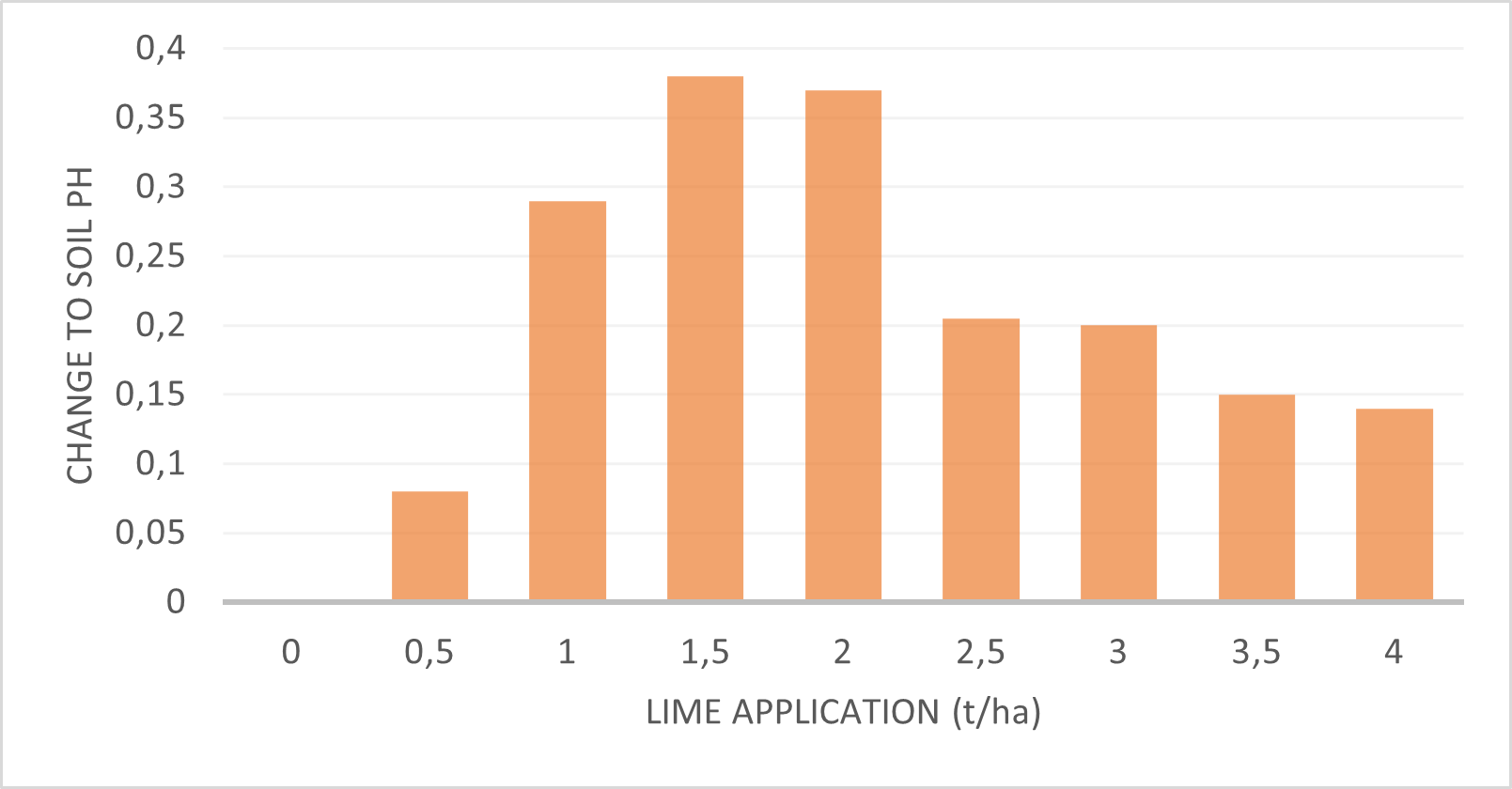
Figure 3: The relationship between the soil pH change to the quantity of lime application (Du Plessis, 2019).
Conventional tillage and moderate nitrogen fertilization cause acidification, equivalent to an annual application of 1 ton of lime per ha (Du Toit, 2019). Lime requires adequate soil moisture to activate the neutralizing reaction, therefore lime works slowly in dry soil. Since neutralization involves a reaction between soil and lime particles, mixing lime with soil increases the efficiency of acidity neutralization.
Alkalinization occurs when the active acidity is neutralised by bicarbonates derived from lime which results in an increase in pH, and when the exchangeable acidity (adsorbed Al3+ and H+-ions) are replaced by basic cations such as Ca2+, Mg2+, K+, and Na+ on the exchange complex. Liming ensures a more favourable soil environment in which plants can thrive. The first step in determining the lime requirement is determining the soil's acidity status by collecting 1-hectare soil samples that are representative across the field. Excessive soil acidity rarely occurs uniformly in a field. Therefore, these parts of the field must be treated differentially according to the liming recommendation. The goal of liming involves the desired outcome which must be achieved according to the specific analysis per area and depth.
Effective liming must meet the following requirements (Noble Research Institute, 1999):
- Accurate determination of the lime requirement and type of lime, considering soil type and crop;
- Accurate and timely application of lime on the field according to the lime requirement;
- Efficient mixing of lime with the soil; and
- Efficient reactivity, chemical purity, and fineness of the lime.
A problem that often occurs in the higher rainfall areas in South Africa is excessive acidity in the subsoil. This type of soil acidity can occur naturally or can be the result of excessive nitrogen fertilization and the failure to lime the topsoil. On soils that do not naturally have acidic subsoils, lime must be incorporated deeply to neutralize the soil acidity at a greater depth, as the calcification effect on the topsoil is transported extremely slowly to the subsoil.
Self-liming is the process where gypsum (CaSO4.2H2O) is used to neutralize subsoil acidity if there are sufficient sesquioxides (iron and aluminium oxides and hydroxides) and 20% or higher clay content in the subsoil. Sufficient lime should be applied to neutralize all the topsoil acidity as well as to reduce the subsoil acidity (Noble Research Institute, 1999).
Conclusion
The solubility and accessibility of plant nutrients are determined by soil pH and are hence the most important soil chemical factor for crop production. Therefore, before the soil's pH is not optimal, other chemical corrections will be inefficient.
Liming leads to low-risk, more economically efficient returns and ultimately a financially sound agricultural industry. A larger investment in lime can then lead to a more reasonable application of fertilizer, with a better yield performance and thus a larger profit margin. There are often different opinions about how soil acidity should be managed. However, it is important that scientific evidence should always serve as the foundation for recommendations.
References
Brady, NC & Weil, RR. 2002. The nature and properties of soils. 13th ed. Prentice Hall: New Jersey.
Du Plessis MJ, 2019. Grondsuurheid. Die produsent se belangrikste bate. Available at: https://sagrainmag.co.za/2019/07/18/grond-die-produsent-se-belangrikste-bate-deel-8-grondsuurheid/ [Accessed 1 November 2022].
Du Toit G, 2019. Grondsuurheid. Bekalking van grond na 'n oes- Suid Afrika. Available at: https://www.farmingportal.co.za/index.php/all-agri-news/nuus-afrikaans/2699-bekalking-van-grond-na-n-oes-suid-afrika [1 November 2022].
Fertasa Fertilizer Handbook, 2018. The Fertilisation Association of South Africa. Lynwood Ridge, Pretoria, South Africa.
Grain SA, 2013. Soil acidity and its management in crop production. Available at: https://www.grainsa.co.za/soil-acidity-and-its-management-in-crop-production. [2 November 2022].
Noble Research Institute, 1999. Understanding and Correcting Soil Acidity. Available at: https://www.noble.org/news/publications/ag-news-and-views/1999/january/understanding-and-correcting-soil-acidity/. [3 November 2022].
Rachel du Preez
 I am an accomplished and knowledgeable BSc Agriculture (Soil Science and Agronomy) Graduate. Attained a degree and honours at the University of the Free State in 2019. I currently work as a Soil Scientist at NWK Limited. I am comfortable working in deadline-driven environments as part of a team or on an individual basis. I possess the ability to take initiative and manage time effectively. My diverse set of skills also covers excellent planning and organising. I consider myself a dedicated and detail orientated individual, who always strives to learn more and to deliver more than expected. I am known for building strong working relationships and I am committed to be a confident and respected member in my environment. I have a farming background and know how things work. I love working with nature and outside in the field. I also have knowledge on some of the difficulties that can be experienced in the agricultural sector.
I am an accomplished and knowledgeable BSc Agriculture (Soil Science and Agronomy) Graduate. Attained a degree and honours at the University of the Free State in 2019. I currently work as a Soil Scientist at NWK Limited. I am comfortable working in deadline-driven environments as part of a team or on an individual basis. I possess the ability to take initiative and manage time effectively. My diverse set of skills also covers excellent planning and organising. I consider myself a dedicated and detail orientated individual, who always strives to learn more and to deliver more than expected. I am known for building strong working relationships and I am committed to be a confident and respected member in my environment. I have a farming background and know how things work. I love working with nature and outside in the field. I also have knowledge on some of the difficulties that can be experienced in the agricultural sector.















